SARS-CoV-2
-
Ngwaọrụ sistemụ siri ike maka SART-Coc-Coagen ngwa ngwa
Renaon 500210 Nkowa 1 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens OnummiriEji eji IkeNnwale sistemụ nke sistemụ syrantigen na-eme ngwa ngwa na teknụzụ immunochromatographyromatographr iji chọpụta sar-Coar-Coragen na Antiga. Ule a na-eji otu ihe na-eji otu ma bu n'obi maka nnwale. A na-atụ aro iji ule a n'ime ụbọchị asaa nke ihe ngosi. A na-akwado ya site na nyocha dinical arụmọrụ. -

SARS-Coc-Coagen ngwa ngwa (NSAL)
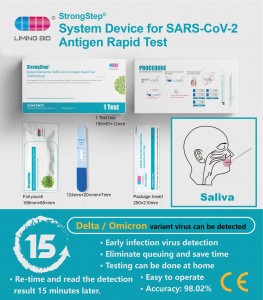
Renaon 500200 Nkowa 1 nnwale / igbe; 5 nnwale / igbe; nnwale 20 / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Awal swab Eji eji Kars-Coragette nke Antigette na-arụ ọrụ na teknụzụ immunochromatotigraphr iji chọpụta sars-2 nucleocen na-egwu Antigen nasal aser na-ahụ n'anya. Nnwale a na-eji naanị ya na-eme naanị maka nnwale. A na-atụgharị ya iji ule a n'ime ụbọchị 5 nke ihe mgbaàmà. Na-akwado ya site na nyocha arụmọrụ. -

SARS-Coc-Coagen ngwa ngwa (iji ya)
Renaon 500200 Nkowa 25 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Awal swab Eji eji Kars-Coragette nke Antigette na-arụ ọrụ na teknụzụ immunochromatotigraphr iji chọpụta sars-2 nucleocen na-egwu Antigen nasal aser na-ahụ n'anya. Nnwale a na-eji naanị ya na-eme naanị maka nnwale. A na-atụgharị ya iji ule a n'ime ụbọchị 5 nke ihe mgbaàmà. Na-akwado ya site na nyocha arụmọrụ. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
SARS-Coc-2 Antigen ngwa ngwa maka mmiri
Renaon 500230 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens OnummiriEji eji Nke a bụ ihe mgbochi a na-eme ngwa ngwa maka nchọpụta nke ihe nkiri SARS-Covleocapen na Antivare na-anakọta ya na ọkpụrụkpụ n'ime ụbọchị ise mbụ nke mgbaàmà. A na-eji ekwenye ekwenye dị ka enyemaka na nchọpụta nke ndọta-19. -

Ngwaọrụ sistemụ maka SARS-Coarv-2 & Perfenza A / B Combo Antigen ngwa ngwa
Renaon 500220 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Nasal / okpeppharngeal swab Eji eji Nke a bụ ihe mgbochi a na-eme ngwa ngwa maka nchọpụta nke ihe nkiri SARS-Covleocapel protein na nke ndị mmadụ na-ahụ maka ụbọchị ise mbụ nke mgbaàmà. A na-eji ekwenye ekwenye dị ka enyemaka na nchọpụta nke ndọta-19. -

Ngwaọrụ Boural Buosasfity System maka SART-CoV-2 Antigen ngwa ngwa
Renaon 500210 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Nasal / okpeppharngeal swab Eji eji Nke a bụ ihe mgbochi a na-eme ngwa ngwa maka nchọpụta nke ihe nkiri SARS-Covleocapel protein na nke ndị mmadụ na-ahụ maka ụbọchị ise mbụ nke mgbaàmà. A na-eji ekwenye ekwenye dị ka enyemaka na nchọpụta nke ndọta-19. -

New coronavirus (SARS-Coc-2) otutu oge pcr
Renaon 500190 Nkowa 96 nnwale / igbe AKWSKWỌ Nchọpụta Nke | Spesitens Nasal / nasopharyngeal swab Eji eji Ebumnuche a ga-eji mee ihe iji nweta ihe nchọpụta swabs swabs swabs swabs swabs swabs, okirikiri site na sistemụ FDA / CE IVD dị n'elu. Ebumnuche a na-ezubere maka ọrụ ndị ọrụ zụrụ azụ
-

SARS-Coc-2 & influenza a / b otutu oge pcr Kit
Renaon 510010 Nkowa 96 nnwale / igbe AKWSKWỌ Nchọpụta Nke | Spesitens Nasal / nasopharyngeal swab / odpharngeal swab Eji eji A na-ebute ọtụtụ ihe na-achọpụta nke ọma na-achọpụta na-achọpụta na-achọpụta ihe na-achọpụta ihe na-achọpụta ihe na-achọpụta ihe na-eweta nke ọma Ma ọ bụ Oropharyngeal Swab Swabo na-eto eto ma ọ bụ obepharngeal swab speens dị iche iche (anakọtara na ntọala ahụike na-agbanwe agbanwe na-agbanwe agbanwe na-ekwekọ - 19 site na ndị na-ahụ maka ahụike ha.
Ebumnuche a na-ezubere maka ọrụ ndị ọrụ zụrụ azụ
-

SARS-Coc-2 IGM / IGG Antodudy ule
Renaon 502090 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Ọbara / ọbara / plasma Eji eji Nke a bụ ihe eji eme ihe eji eme ihe eji eme ihe eji achọ ime ụlọ, ọgwụ igg na-acha uhie uhie na cors-CoV-2 na ọbara ọ bụla, ọbara ma ọ bụ plasma. Ule a pere mpe na US ka nkesa nke na-ekesara na Clika iji mee nnwale dị oke egwu.
Nnwale a abụghị nke FDA.
Nsonaazụ ọjọọ anaghị egbochi nnukwu sars-vive.
Ekwesighi iji nsonaazụ na-anwale nnwale iji chọpụta ma ọ bụ wezuga nnukwu sars-vive.
Nsonaazụ dị mma nwere ike ịbụ ọrịa gara aga ma ọ bụ nke na-efe efe na-enweghị ntụpọ na Coronavirus, dị ka coronavirus hku1, NL63, OC43, ma ọ bụ 229e.







