Ngwaahịa
-

Ule Toxooplasma Gondii Antigen ngwa ngwa
Renaon 500420 Nkowa 1,20 nnwale / igbe AKWSKWỌ Nchọpụta Ahuhu Spesitens Ihe dị mkpa (cat / nkịta) Eji eji A na-eji ngwaahịa a mee ihe maka nyocha nke nkịta na ihe nlele faecal maka Toxooplasma Gondii Antigen ma nwee ike iji ọrịa Toxoplasma nke Toxoplasma. -

Ngwaọrụ sistemụ maka ọrịa na-efe efe (Feline Herpesvirus & Filine Calicivirus) combo antigen ngwa ngwa
Renaon 500430 Nkowa 1,20 nnwale / igbe AKWSKWỌ Nchọpụta Ahuhu Spesitens Nasal swab (cat) Eji eji A na-eji ngwaahịa a mee ihe ngwa ngwa nke ihe nlere anya cat cencer na nke nasal conproonsvirus na Anine cuprovirus cuprovirus nke ọrịa feline helinirus. -

Ngwaọrụ sistemụ maka ọrịa Felin (Filine Parvovirus & Filine Coronavirus) combo antigen ngwa ngwa
Renaon 500440 Nkowa 1,20 nnwale / igbe AKWSKWỌ Nchọpụta Ahuhu Spesitens Ihe dị mkpa (Cat) Eji eji Ọrịa Feline Divestic / Filine Coronavic Kit (Latex Immunomatomatic Kit (Latex Immunomatos) na-eji mmeghachi omume nke nje na-egbochi ọrịa Filine / Filine coronavirus na swab. -

Ule Gardia Lamblia Antigen ngwa ngwa
Renaon 501100 Nkowa 1,20 nnwale / igbe AKWSKWỌ Nchọpụta Ahuhu Spesitens Ihe dị mkpa (cat / nkịta) Eji eji A na-eji ngwaahịa a mee ihe maka nyocha nke nkịta na ihe nlele maka GARLALA Lamblia Antigen, enwere ike iji ya dị ka ihe enyemaka na nchọta nke Garlia Lamblia. -

Nchọpụta nke Pet
Ngwaahịa nchọpụta na-achọpụta na ntanetị.
Biko kpọtụrụ anyị ma ịchọrọ ya.
Nina Wang:sales@limingbio.com
Vicky Chen:vickychen@limingbio.com
-

Dermatyosis ngwa ngwa
Renaon 500280 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Swab / ntu / scurf / ntutu Eji eji Kit siri ike na-ahụ maka ngwa ngwa na-ahụ maka nchọpụta nke α-1, 6 Mannose na dịkwa ka usoro ọ bụla. Ebumnuche a na-ezubere ka a ga-eme dị ka enyemaka na nyocha nke Dermatophytosis. -

Antida Albicans / Trichomonas Andernis / Gardreella vantitis antigen ngwa ngwa
Renaon 500340 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Vanvovaginal calrisis / Trichomonal vadinitis / nje bacterias Eji eji Maka nchọpụta tozuru etozu nke Trichmonas na / ma ọ bụ candida na / ma ọ bụ Gardreella Vazọ sitere na Swabs Swabs ma ọ bụ site na nnu swabs site na Ugwu mmiri site na Ugwu Swabs. Ebumnuche a na-ezubere iji mee ihe enyemaka dị ka ihe enyemaka nke Kọrọda Algicans na / ma ọ bụ Trichmonas AnvigarSel na ọrịa. -

Ule Gardella vandernis antigen ngwa ngwa
Renaon 500330 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Mkpụrụ vaịn Eji eji Maka nchọpụta ruru eru nke Gardneela verpanizes site na swabs ma ọ bụ site na usoro nnu dị njikere mgbe ị na-eme Ugwu mmiri site na Ugwu mmiri site na Ugwu mmiri site na Ugwu mmiri site na Ugwu mmiri. Ebumnuche a na-ezubere ka a ga-eji ya dị ka ihe enyemaka na nchọta nke Gardneel dere na-efe efe. -
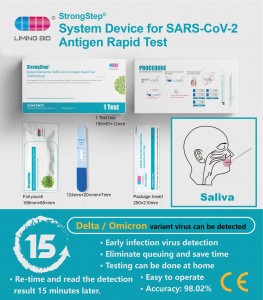
Ngwaọrụ sistemụ siri ike maka SART-Coc-Coagen ngwa ngwa
Renaon 500210 Nkowa 1 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens OnummiriEji eji IkeNnwale sistemụ nke sistemụ syrantigen na-eme ngwa ngwa na teknụzụ immunochromatographyromatographr iji chọpụta sar-Coar-Coragen na Antiga. Ule a na-eji otu ihe na-eji otu ma bu n'obi maka nnwale. A na-atụ aro iji ule a n'ime ụbọchị asaa nke ihe ngosi. A na-akwado ya site na nyocha dinical arụmọrụ. -

SARS-Coc-Coagen ngwa ngwa (NSAL)
Renaon 500200 Nkowa 1 nnwale / igbe; 5 nnwale / igbe; nnwale 20 / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Awal swab Eji eji Kars-Coragette nke Antigette na-arụ ọrụ na teknụzụ immunochromatotigraphr iji chọpụta sars-2 nucleocen na-egwu Antigen nasal aser na-ahụ n'anya. Nnwale a na-eji naanị ya na-eme naanị maka nnwale. A na-atụgharị ya iji ule a n'ime ụbọchị 5 nke ihe mgbaàmà. Na-akwado ya site na nyocha arụmọrụ. -

SARS-Coc-Coagen ngwa ngwa (iji ya)
Renaon 500200 Nkowa 25 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens Awal swab Eji eji Kars-Coragette nke Antigette na-arụ ọrụ na teknụzụ immunochromatotigraphr iji chọpụta sars-2 nucleocen na-egwu Antigen nasal aser na-ahụ n'anya. Nnwale a na-eji naanị ya na-eme naanị maka nnwale. A na-atụgharị ya iji ule a n'ime ụbọchị 5 nke ihe mgbaàmà. Na-akwado ya site na nyocha arụmọrụ. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
SARS-Coc-2 Antigen ngwa ngwa maka mmiri
Renaon 500230 Nkowa 20 nnwale / igbe AKWSKWỌ Nchọpụta Imumumatogratogratogratogratographic Spesitens OnummiriEji eji Nke a bụ ihe mgbochi a na-eme ngwa ngwa maka nchọpụta nke ihe nkiri SARS-Covleocapen na Antivare na-anakọta ya na ọkpụrụkpụ n'ime ụbọchị ise mbụ nke mgbaàmà. A na-eji ekwenye ekwenye dị ka enyemaka na nchọpụta nke ndọta-19.







